Upholding high research standards in pharmacoepidemiology and pharmacovigilance based on the principles of scientific independence, transparency, and use of robust methodologies, is at the core of ENCePP. The ENCePP Seal was introduced in 2010 to recognise studies following these core principles, conducted in line with the rules and requirements for independent and transparent research laid down in the ENCePP Code of Conduct and in established international guidelines, and taking into account the standards described in the ENCePP Guide on Methodological Standards in Pharmacoepidemiology.
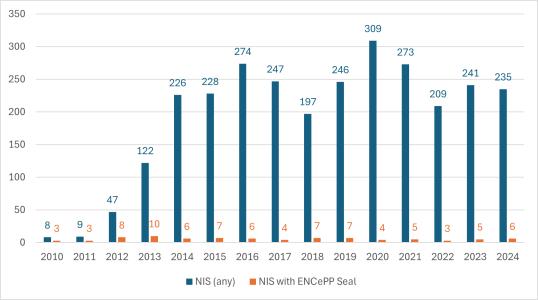
However, uptake of the Seal has been very limited. Since its launch, only 84 non-interventional studies (NIS) with a Seal have been registered in the EU PAS Register and the ensuing HMA-EMA Catalogue of real-world data studies (Figure 1).
Figure 1 – number of NIS and ENCePP Seals, by year, 2010-2024
Based on experience over time, the changing NIS landscape, the recent results of qualitative research undertaken under the 2024-2026 ENCePP workplan, and reflection at Steering Group level, the Steering Group made the decision in January 2025 to discontinue the use of the Seal.
Its individual components, namely, the Code of Conduct, the Methods Guide, and the Checklist for Study Protocols, remain important tools reflecting the ENCePP core principles, and researchers are strongly encouraged to continue using them when implementing studies. All relevant documentation and systems (such as the above-mentioned ENCePP tools, the ENCePP website, and the HMA-EMA Catalogues of real-world data sources and studies) will be updated to reflect this change. Studies which already have the Seal are not impacted and will retain their status in the RWD Catalogues.
Applying its core principles, ENCePP will continue promoting excellence in pharmacovigilance and pharmacoepidemiology, and supporting networking, exchange of good practice, and discussions on cutting-edge science. Through a collaborative approach to excellence in non-interventional research, ENCePP can collectively support getting medicines to patients faster, and optimise their safe and effective use.
Details
- Publication date
- 18 February 2025
- Author
- European Medicines Agency